About NCATS
Congress established NCATS in 2011 with an extremely important mission --to transform scientific discoveries into new treatments and cures that can be delivered faster to patients by catalyzing the generation of innovative methods and technologies that enhance and accelerate the development, testing and implementation of diagnostics and therapeutics across a wide range of human diseases. Its goal by doing so is to get more treatments to more patients more quickly.
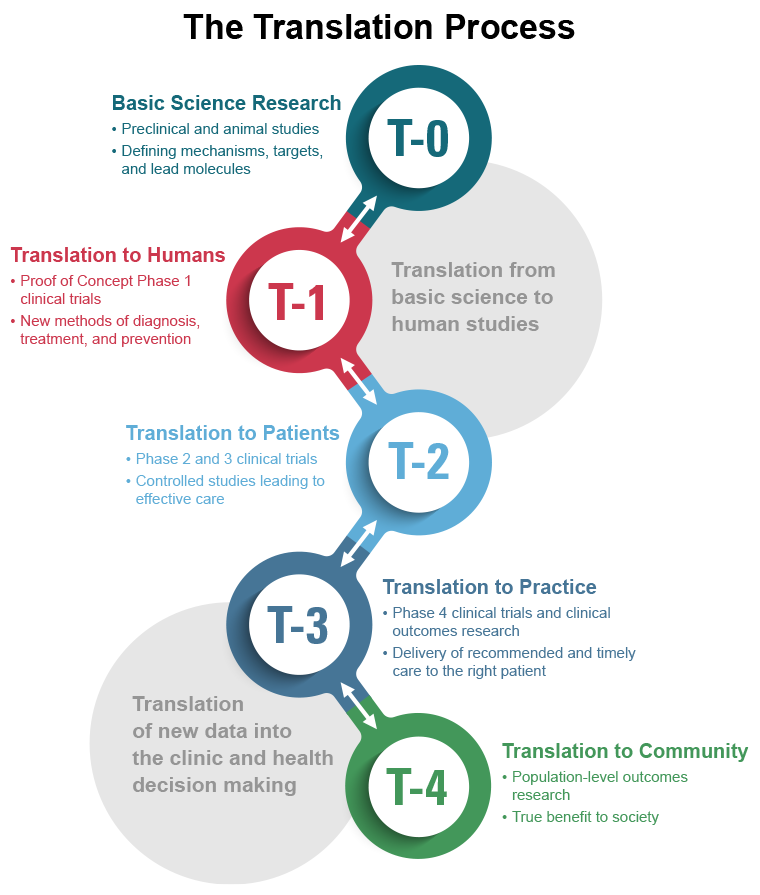
Successful therapeutic development occurs in three steps, beginning with basic-science discoveries and culminating in the regulatory assessment of data from clinical trials in humans. It is in the middle step of this process – the translational work required to determine if the discovery “candidate” has a chance of being “druggable” and testing it in various non-human systems to assess its potential safety and effectiveness – that the major bottlenecks occur. It is in this translational phase that long timelines (about 15 years), steep costs (billions of dollars), and high failure rates (about 95%) take their toll. NCATS-supported researchers seek to advance the science of translation by identifying the bottlenecks in the therapeutic development pipeline that may be amenable to reengineering; experimenting with innovative approaches to reduce, remove, or bypass these bottlenecks; and evaluating these innovations by assessing their performance in real-world applications. All of this is being done in a transparent scientific environment to ensure that information about successes—and failures—is made swiftly available to all stakeholders.
NCATS Programs and Initiatives
NCATS oversees 33 distinct programs and initiatives. Some highlights below:
- Gene Therapy – NCATS is working collaboratively with all the stakeholders to remove the bottlenecks and resolve the issues slowing the progress in all gene therapy programs. The approaches taken in these programs help speed the development of treatments for multiple rare diseases at a time. Some specific gene therapy-related programs include:
- The Platform Vector Gene Therapy (PaVe-GT) pilot project – This pilot seeks to increase efficiency of clinical trial startup by using the same gene delivery system and manufacturing methods for multiple rare disease gene therapies.
- Bespoke Gene Therapy Consortium (BGTC) – a public-private partnership that aims to develop platforms and standards that will speed up the development of delivery of customized, or “bespoke,” gene therapies that could treat the millions of people affected by rare disease.
- Tissue Chips for Drug Screening Program – A lot of promising medications fail to be safe and effective in human clinical trials despite promising preclinical studies. NCATS along with other NIH Centers and Institutes, FDA, and NASA are addressing this problem through the Tissue Chips for Drug Screening Program. This program uses tissue chips, also called organs on chips, that are built from human cells to mimic the structure and function of human hearts, kidneys, lungs and other organ systems to test the potential effects of drugs on human tissue. These tissue chips are testing the potency of investigational drugs in a faster and more effective way than current methods.
- 3-D Tissue Bioprinting Program – NCATS experts are using 3-D bioprinting technology to develop tissue models that mimic the cells and tissue of living organisms to better predict the effectiveness and toxicity of potential drugs in humans. This technology can help to streamline the drug development process by helping researchers more quickly understand how an investigational drug may interact with the human body.
- Clinical and Translational Science Awards (CTSA) Program – The CTSA program supports a national network of medical research institutions that work together to improve the translational research process.
- National COVID Cohort Collaborative (N3C) – The N3C program is the largest collection of secure and deidentified clinical data in the United States for COVID-19 research. This repository of COVID-19 data is continuously growing and allows researchers and clinicians to study COVID-19 health outcomes.
NCATS Resources
A Rare Public Health Challenge
Originally posted at directorsblog.nih.gov on by Joni Rutter, Ph.D., National Center for Advancing Translational Sciences.
Most public health challenges may seem obvious. The COVID-19 pandemic, for example, swept the globe and in some way touched the lives of everyone. But not all public health challenges are as readily apparent.
Rare diseases are a case in point. While individually each disease is rare, collectively rare diseases are common: More than 10,000 rare diseases affect nearly 400 million people worldwide. In the United States, the prevalence of rare diseases (over 30 million people) rivals or exceeds that of common diseases such as diabetes (37.3 million people), Alzheimer’s disease (6.5 million people), and heart failure (6.2 million people).